- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Obeticholic Acid may Improve outcomes in NASH-Related Liver Fibrosis
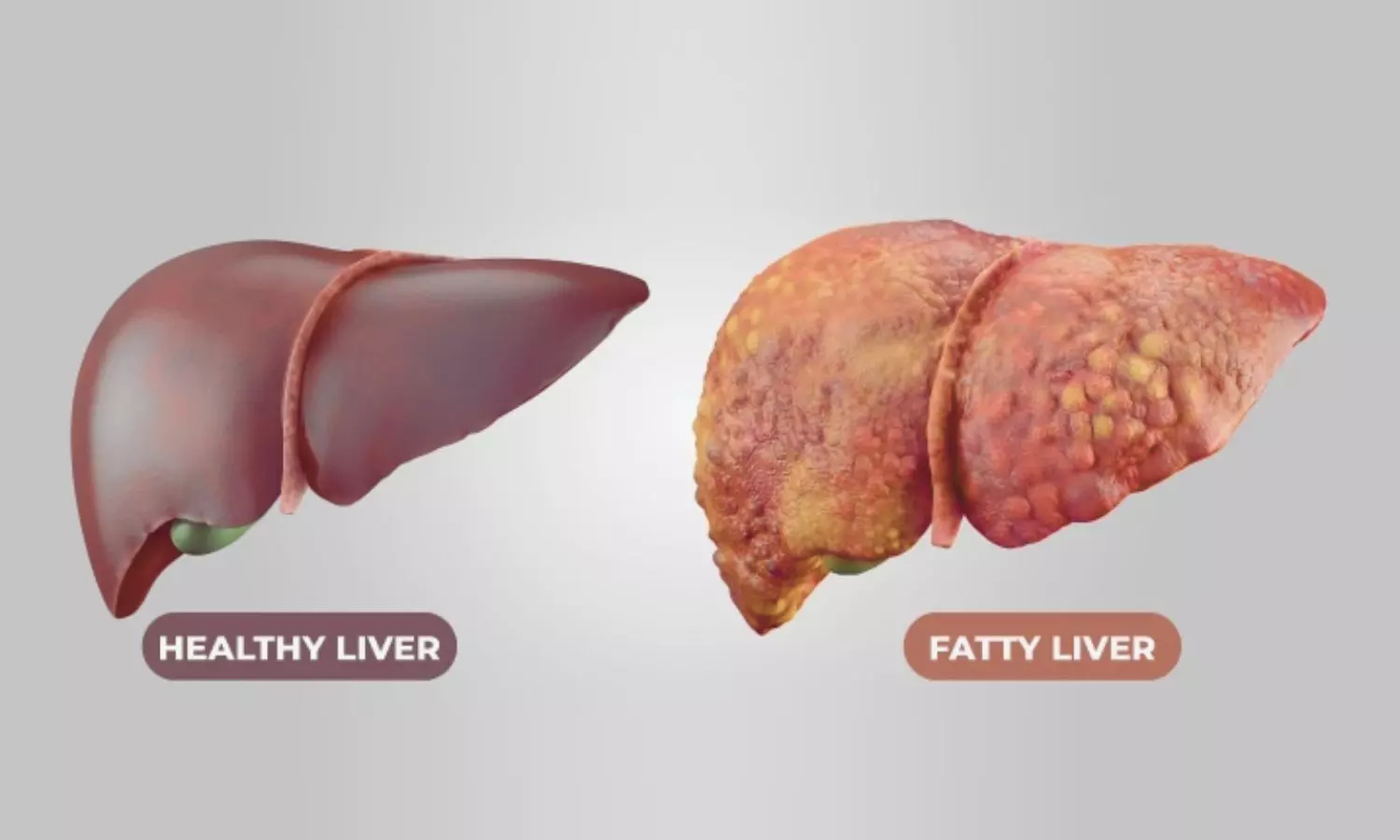
A new study published in Journal of Hepatology confirms the efficacy of Obeticholic Acid (OCA), a novel farnesoid X receptor agonist and antifibrotic agent against non-alcoholic steatohepatitis (NASH).
The Phase III REGENERATE trial originally reported results from an 18-month liver biopsy analysis involving OCA. The treatment demonstrated its efficacy, prompting further investigation. In this study, the potential of OCA was reaffirmed, providing hope for those battling this debilitating liver condition.
The study aimed to validate the previous findings and gather additional data to support OCA's effectiveness. A diverse panel of three pathologists independently evaluated digitized whole-slide images using the NASH Clinical Research Network scoring system. The primary endpoints were either an improvement of at least one stage in fibrosis without worsening of NASH or NASH resolution without worsening of fibrosis.
Among 931 participants, OCA demonstrated its potential with 22.4% achieving an improvement in fibrosis without NASH worsening, compared to only 9.6% on a placebo. Furthermore, 6.5% of those on OCA achieved NASH resolution without fibrosis progression, compared to 3.5% on the placebo.
Not only did OCA showcase its efficacy, but it also demonstrated a high degree of safety and tolerability. The study included data from 2,477 participants, and the incidence of treatment-emergent adverse events (TEAEs) and serious TEAEs was not substantially different across treatment groups. The most common TEAE reported was pruritus. Moreover, rates of hepatic, renal, and cardiovascular events were low and comparable across treatment groups.
These findings highlight OCA's potential to significantly impact the lives of patients with NASH-related liver fibrosis. Preventing fibrosis from progressing to cirrhosis or even reversing it is a critical goal in NASH treatment. OCA’s antifibrotic effects, proven effectiveness, and favorable safety profile offer hope for patients dealing with this challenging condition.
Source:
Sanyal, A. J., Ratziu, V., Loomba, R., Anstee, Q. M., Kowdley, K. V., Rinella, M. E., Knapple, W., Lawitz, E. J., Abdelmalek, M. F., Shiff, S. J., Sawhney, S., Capozza, T., … Younossi, Z. M. (2023). Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. Journal of Hepatology, 79(5), 1110–1120. https://doi.org/10.1016/j.jhep.2023.07.014
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in


