- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Heat shock protein 90 inhibition effective and safe option against Hidradenitis Suppurativa
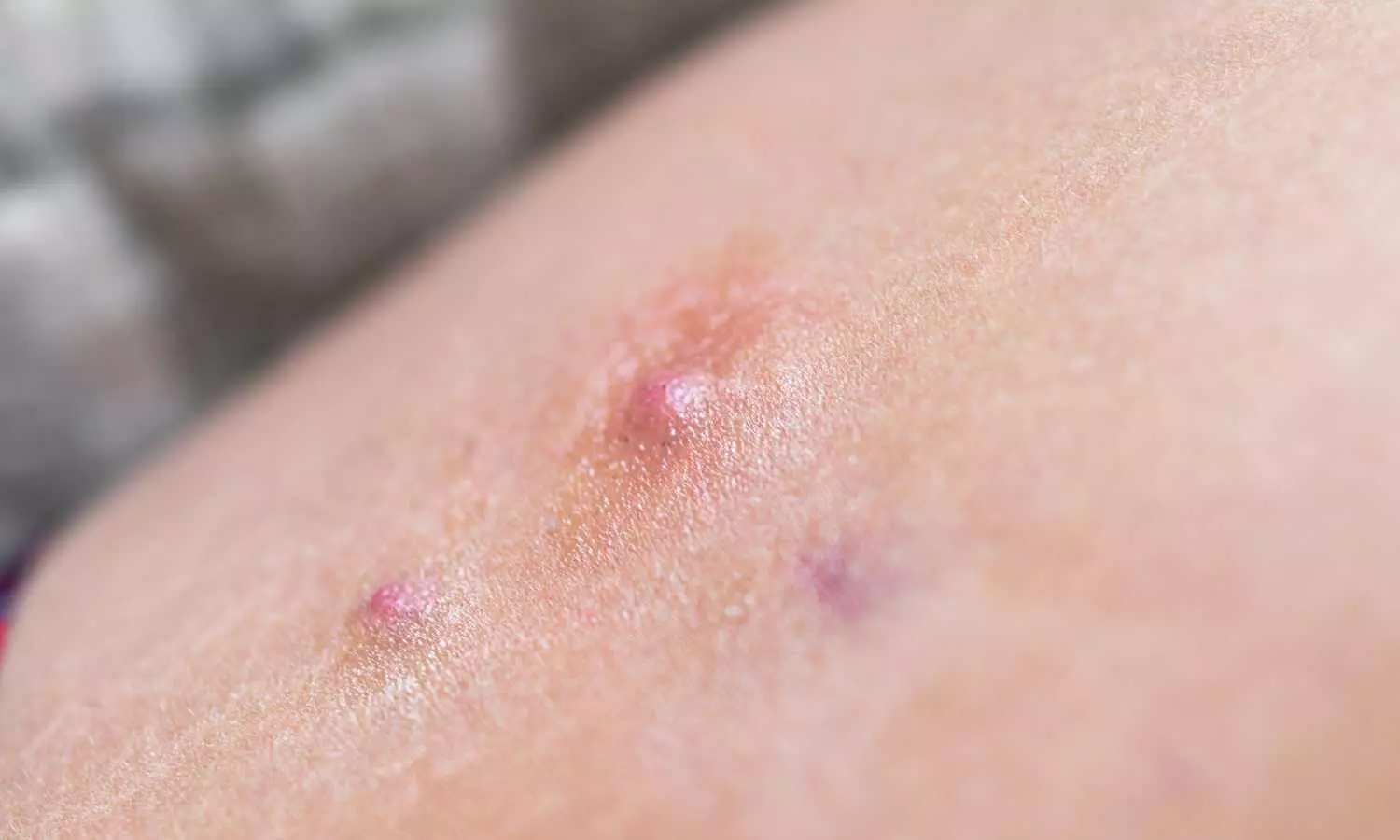
In a recent clinical trial discovered promising outcomes of heat shock protein 90 inhibition by RGRN-305 for individuals suffering from moderate to severe Hidradenitis Suppurativa (HS), a painful immune-mediated disorder. This results were published in Journal of American Medical Association.
The randomized clinical study was conducted from September 22, 2021, to August 29, 2022 at the Department of Dermatology, Aarhus University Hospital in Denmark. The study included 15 patients who met eligibility criteria-aged 18 or older with moderate to severe HS and aimed to evaluate the feasibility of using RGRN-305, a heat shock protein 90 inhibitor in treating the disorder.
The patients randomly received either oral RGRN-305, a 250-mg tablet, or a matching placebo once daily for 16 weeks. The primary efficacy endpoint was the percentage of patients achieving Hidradenitis Suppurativa Clinical Response 50 (HiSCR-50) at week 16.
The trial demonstrated that the RGRN-305 group outperformed the placebo group in achieving HiSCR-50 at week 16, with 60% compared to 20%, respectively. Secondary endpoints included HiSCR-75 and HiSCR-90, Dermatology Life Quality Index scores, and a pain numeric rating scale, also showed significant improvements in the RGRN-305 group.
The safety of RGRN-305 was significant in the study, with no deaths or serious adverse events reported. Treatment-emergent adverse events were equally distributed between the RGRN-305 and placebo groups which indicated that this treatment was well-tolerated.
These highlights suggest that heat shock protein 90 inhibition by RGRN-305 presents a promising mechanism of action against Hidradenitis Suppurativa. The success of this trial underscores the importance of further evaluation in larger-scale cohorts to improve and ease the quality of life of individuals with this painful immune disorder.
Source:
Ben Abdallah, H., Bregnhøj, A., Emmanuel, T., Ghatnekar, G., Johansen, C., & Iversen, L. (2023). Efficacy and Safety of the Heat Shock Protein 90 Inhibitor RGRN-305 in Hidradenitis Suppurativa. In JAMA Dermatology. American Medical Association (AMA). https://doi.org/10.1001/jamadermatol.2023.4800
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in


